
Cancer Vaccines Market on Track for Major Expansion in the 7MM Amid Immunotherapy Advancements, Predicts DelveInsight
The cancer vaccine market is anticipated to experience substantial growth in the coming years. This growth is driven by the rising number of cancer diagnoses, heightened awareness of cancer vaccines, and the growing pipeline of cancer vaccines currently in clinical trials or awaiting approval from various companies.
/EIN News/ -- New York, USA, Feb. 17, 2025 (GLOBE NEWSWIRE) -- Cancer Vaccines Market on Track for Major Expansion in the 7MM Amid Immunotherapy Advancements, Predicts DelveInsight
The cancer vaccine market is anticipated to experience substantial growth in the coming years. This growth is driven by the rising number of cancer diagnoses, heightened awareness of cancer vaccines, and the growing pipeline of cancer vaccines currently in clinical trials or awaiting approval from various companies.
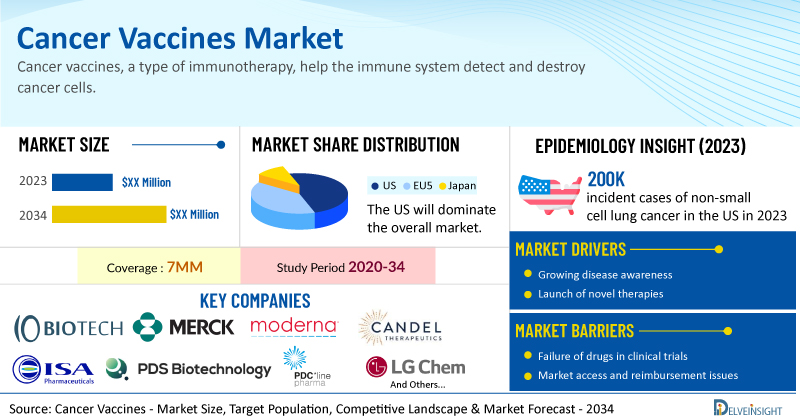
DelveInsight’s Cancer Vaccines Market Size, Target Population, Competitive Landscape & Market Forecast report includes a comprehensive understanding of current treatment practices, emerging cancer vaccines, market share of individual therapies, and current and forecasted cancer vaccines market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Cancer Vaccines Market Report
- As per DelveInsight’s analysis, the cancer vaccines market is expected to grow significantly in the 7MM by 2034.
- The estimated incident cases of non-small cell lung cancer in the US in 2023 were nearly 200K.
- Leading cancer vaccine companies such as IO Biotech, Merck, Moderna, Candle Therapeutics, ISA Pharmaceuticals, PDS Biotechnology, PDC*line Pharma, LG Chem, AVEO Oncology, Archival Farma, and others are developing novel cancer vaccines that can be available in the cancer vaccines market in the coming years.
- Some of the key cancer vaccines in the pipeline include IO102-IO103, mRNA-4157 (V940) + KEYTRUDA, CAN-2409, Nelipepimut-S + Cemiplimab, PDS0101 + Pembrolizumab, PDC*lung01, LB-LR1109, RUTI, and others.
- In July 2024, IO Biotech announced that data from one of the Phase II basket trials of IO102-IO103 in combination with pembrolizumab will be presented at the ESMO Congress 2024. The data for the complete squamous cell carcinoma of the head and neck (SCCHN) cohort of the IOB-022/KN-D38 study.
- In June 2024, LG Chem and AVEO Oncology announced that it has enrolled the first patient in the US for a Phase I clinical study of LB-LR1109 (NCT06332755; LG project code LR19155), LG Chem’s first proprietary anti-cancer investigational drug candidate, in participants with unresectable and NSCLC, head and neck squamous cell carcinoma, RCC, urothelial carcinoma, or malignant melanoma.
- In May 2024, Candel Therapeutics reported prolonged overall survival in Phase II Trial of CAN-2409 for NSCLC at the 2024 ASCO annual meeting.
- In April 2024, CAN-2409 was granted Orphan Drug Designation by the US FDA for the treatment of pancreatic cancer.
Discover which therapies are expected to grab the cancer vaccines market share @ Cancer Vaccines Market and Competitive Landscape Report
Cancer Vaccines Market Dynamics
The cancer vaccine market is emerging as a promising segment within the biopharmaceutical industry, driven by both technological advancements and increasing demand for innovative cancer treatments. Traditional cancer therapies, such as chemotherapy and radiation, have proven effective in many cases but often come with significant side effects. As a result, cancer vaccines, which are designed to stimulate the immune system to target and destroy cancer cells, are gaining significant attention. This growth is bolstered by increasing research into tumor-specific antigens, personalized medicine, and the ability to develop vaccines that target multiple cancer types.
One of the key dynamics of the cancer vaccine market is the shift towards immunotherapy. Immuno-oncology therapies, including cancer vaccines, are increasingly seen as complementary to existing treatments. While there are currently only a few approved cancer vaccines on the market, the pipeline for new candidates is expanding rapidly, particularly with advancements in messenger RNA (mRNA) technology. The success of COVID-19 vaccines has catalyzed interest and investment in mRNA technology for cancer treatment, which has led to the accelerated development of personalized vaccines that can target specific mutations in a patient's cancer cells.
The competitive landscape is becoming increasingly complex as both established pharmaceutical companies and biotech startups vie for market share. Large companies like Merck, GlaxoSmithKline, and Moderna are heavily investing in cancer vaccine research and development, with some already making significant strides toward approval of their therapies. Smaller biotech companies are also pushing the envelope by focusing on niche markets or innovative approaches to vaccine delivery and tumor targeting. This competitive environment is fostering both collaboration and consolidation, as companies partner to combine their expertise in immunotherapy, genomics, and vaccine technologies.
Despite the promising outlook, there are several challenges that the cancer vaccine market faces. High development costs, regulatory hurdles, and patient-specific variations in response to therapy present significant barriers. Moreover, the complexity of designing vaccines that are effective across different types of cancer or even individual patients requires continued investment in personalized medicine. Nevertheless, the potential for cancer vaccines to provide more targeted, less toxic treatments remains a major driving force behind the market’s rapid evolution.
Overall, the cancer vaccine market is poised for continued growth as technology advances, regulatory landscapes evolve, and more effective therapies are brought to market. With increasing collaboration between industry stakeholders and continued innovation, the future holds great promise for the development of vaccines that can revolutionize cancer treatment and offer patients more effective, long-term solutions.
Cancer Vaccines Treatment Market
Cancer vaccines, which have been developed over several decades, differ from traditional vaccines aimed at infectious diseases. While preventive vaccines are given to healthy individuals, therapeutic cancer vaccines are designed to enhance the immune system's ability to target and destroy cancer cells. Bacillus Calmette-Guérin (BCG) was the first FDA-approved immunotherapy and is still used for early-stage bladder cancer. IMLYGIC from Amgen received FDA approval in 2015, following PROVENGE from Dendreon Pharmaceuticals in 2010.
PROVENGE (sipuleucel-T), developed by Dendreon, is an autologous cellular immunotherapy administered via intravenous infusion. It is made from the patient’s own peripheral blood mononuclear cells, including antigen-presenting cells (APCs), which are activated in a culture with a recombinant protein, PAP-GM-CSF. This protein combines prostatic acid phosphatase (PAP), an antigen found in prostate cancer, with granulocyte-macrophage colony-stimulating factor (GM-CSF), which activates immune cells.
Despite its innovation, PROVENGE faced market challenges due to its high cost and reimbursement difficulties. Similarly, IMLYGIC, despite being newer, encountered obstacles because of its intratumoral injection method and the competition from immune checkpoint inhibitors (CPIs), which are more effective and have fewer side effects. These factors resulted in limited revenue for both therapies.
Learn more about the FDA-approved cancer vaccines @ Cancer Vaccines
Key Emerging Cancer Vaccines and Companies
Some of the vaccines in the pipeline include IO102-IO103 (IO Biotech), CAN-2409 (Candel Therapeutics), mRNA-4157 (V940) + KEYTRUDA (Merck and Moderna), PDC*lung01 (PDC*line Pharma/LG Chem), and LB-LR1109 (LG Chem and AVEO Oncology), among others.
IO102-IO103 is an experimental cancer vaccine designed to target and eliminate both tumor cells and immune-suppressive cells within the tumor microenvironment (TME). It works by stimulating T-cell activation and expansion against indoleamine 2,3-dioxygenase (IDO) and/or programmed death-ligand 1 (PD-L1) cells. The company is currently conducting a pivotal Phase III trial (IOB-013/KN-D18; NCT05155254) to evaluate the combination of IO102-IO103 with pembrolizumab in patients with advanced melanoma. Additionally, the vaccine combination is being tested in two Phase II basket trials: one (NCT05077709) in patients with solid tumors, and another (NCT05280314) as a neo-adjuvant/adjuvant treatment for patients with solid tumors.
CAN-2409 is an experimental, off-the-shelf, replication-defective adenovirus engineered to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene directly to a patient's tumor, aiming to trigger a systemic anti-tumor immune response. Candel is currently assessing the impact of CAN-2409 treatment in NSCLC borderline resectable PDAC and localized non-metastatic prostate cancer.
In April 2024, CAN-2409 received Orphan Drug Designation from the US FDA for the treatment of pancreatic cancer. It was also granted Fast Track Designation by the FDA for treating PDAC and stage III/IV NSCLC. During the 2024 ASCO Annual Meeting in May, Candel Therapeutics reported significant improvement in overall survival from a Phase II trial of CAN-2409 for NSCLC. Additionally, in April 2024, the company shared positive interim results from the Phase II clinical trial of CAN-2409 in non-metastatic pancreatic cancer.
mRNA-4157 (V940) is an innovative experimental mRNA-based personalized neoantigen therapy (INT) that uses synthetic mRNA to encode up to 34 neoantigens. This therapy is tailored to the patient's tumor's specific mutational profile. Developed by Moderna in partnership with Merck, the mRNA-4157 program demonstrates promising clinical outcomes, with ongoing Phase III trials targeting high-risk melanoma and non-small cell lung cancer. Additionally, three new randomized clinical trials were launched in 2024, focusing on kidney cancer, high-risk muscle-invasive bladder cancer, and cutaneous squamous cell carcinoma.
The anticipated launch of these emerging vaccines are poised to transform the cancer vaccine market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the cancer vaccines market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about cancer vaccine clinical trials, visit @ Cancer Vaccines in Development
Cancer Vaccines Overview
Cancer vaccines, a type of immunotherapy, help the immune system detect and destroy cancer cells. These vaccines can be derived from a patient's tumor cells, dendritic cells, or specific proteins found in cancer cells. They are classified into two categories: preventive and therapeutic. Preventive vaccines are more widely recognized and are aimed at preventing viral infections that may lead to future cancers. On the other hand, therapeutic vaccines target existing cancer, addressing various cancer types, locations, and even personalized antigens specific to an individual’s cancer.
Cancer treatment vaccines work by boosting the body’s natural defense mechanisms against cancer. Unlike preventive vaccines, which are used to avoid cancer-causing agents, treatment vaccines are designed for individuals already diagnosed with cancer. They target cancer cells directly, focusing on tumor-associated antigens that are unique to cancer cells and often absent or present at lower levels in normal cells. These vaccines help the immune system identify and attack these antigens, leading to the destruction of cancer cells.
Cancer Vaccines Epidemiology Segmentation
Uveal melanoma affects between 500 and 600 patients in the UK every year. Children in the age group 0-14 have been observed to have an incident rate of nearly 15 for neuroblastoma in Japan. The cancer vaccines market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of Selected Indications
- Total Eligible Patient Pool for Cancer Vaccines in Selected Indications
- Total Treated Cases in Selected Indications for Cancer Vaccines
Download the report to understand what epidemiologists are saying about cancer vaccine patient trends in 7MM @ Cancer Vaccines Patient Pool
| Cancer Vaccines Report Metrics | Details |
| Study Period | 2020–2034 |
| Cancer Vaccines Report Coverage | 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Cancer Vaccine Companies | IO Biotech, Merck, Moderna, Candle Therapeutics, ISA Pharmaceuticals, PDS Biotechnology, PDC*line Pharma, LG Chem, AVEO Oncology, Archival Farma, Merck Sharp & Dohme, BioVex, Dynavax Technologies, Dendreon Pharmaceuticals, and others |
| Key Vaccines for Cancer Prevention and Treatment | IO102-IO103, mRNA-4157 (V940) + KEYTRUDA, CAN-2409, Nelipepimut-S + Cemiplimab, PDS0101 + Pembrolizumab, PDC*lung01, LB-LR1109, RUTI, GARDASIL 9, IMLYGIC, HEPLISAV-B, PROVENGE, and others |
Scope of the Cancer Vaccines Market Report
- Cancer Vaccines Therapeutic Assessment: Cancer Vaccines current marketed and emerging therapies
- Cancer Vaccines Market Dynamics: Conjoint Analysis of Emerging Cancer Vaccines Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Cancer Vaccines Market Access and Reimbursement
Discover more about Cancer Vaccines drugs in development @ Cancer Vaccines Clinical Trials
Table of Contents
| 1. | Cancer Vaccines Market Key Insights |
| 2. | Cancer Vaccines Market Report Introduction |
| 3. | Executive Summary of Cancer Vaccines |
| 4. | Key Events |
| 5. | Cancer Vaccines Market Forecast Methodology |
| 6. | Cancer Vaccines Market Overview at a Glance in the 7MM |
| 7. | Cancer Vaccines: Background and Overview |
| 8. | Cancer Vaccines Target Patient Pool |
| 9. | Cancer Vaccines Marketed Drugs |
| 10. | Cancer Vaccines Emerging Drugs |
| 11. | Seven Major Cancer Vaccines Market Analysis |
| 12. | Cancer Vaccines Market Access and Reimbursement |
| 13. | SWOT Analysis |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | Appendix |
| 17. | DelveInsight Capabilities |
| 18. | Disclaimer |
| 19. | About DelveInsight |
Related Reports
Cancer Vaccines Competitive Landscape
Cancer Vaccines Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cancer vaccine companies, including Imvax, Transgene, BrightPath Biotherapeutics, Vaccitech, Amal Therapeutics, Enterome, Moderna, Inc., Ultimovacs ASA, OSE Immunotherapeutic, IO Biotech, PDS Biotech, LIKANGLIFE SCIENCES, ISA Pharmaceuticals, Voltron Therapeutics, Inc., RNAImmune, among others.
Neoantigen-based Personalized Cancer Therapeutic Vaccines Market
Neoantigen-based Personalized Cancer Therapeutic Vaccines Competitive Landscape and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key neoantigen-based personalized cancer therapeutic vaccine companies, including IO Biotech, Merck, Moderna, Candle Therapeutics, ISA Pharmaceuticals, PDS Biotechnology, PDC*line Pharma, LG Chem, AVEO Oncology, Archival Farma, Merck Sharp & Dohme, BioVex, Dynavax Technologies, Dendreon Pharmaceuticals, among others.
Neoantigen-Based Personalized Cancer Therapeutic Vaccine Pipeline
Neoantigen-Based Personalized Cancer Therapeutic Vaccine Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key neoantigen-based personalized cancer therapeutic vaccine companies including OSE Immunotherapeutics, Genocea Biosciences, Hangzhou Neoantigen Therapeutics, among others.
mRNA Vaccines And Therapeutics Market
mRNA Vaccines And Therapeutics Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key mRNA vaccines and therapeutics companies, including Pfizer Inc., BioNTech SE, Moderna, Inc., Gennova Biopharmaceuticals Limited, GSK plc., Daiichi Sankyo, Arcturus, Boehringer Ingelheim International GmbH, Ethris GmbH, CureVac SE, AIM Vaccine Corporation, Charoen Pokphand Group, Argos Therapeutics Inc., Sanofi, Kernal Biologics Inc, among others.
Non-Small Cell Lung Cancer Market
Non-Small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NSCLC companies, including EMD Serono, Merck, Cellular Biomedicine Group, Inc., Celgene, CellSight Technologies, Inc., BeyondSpring Pharmaceuticals Inc., J Ints Bio, Forward Pharmaceuticals Co., Ltd., AstraZeneca, Bristol-Myers Squibb, Teligene US, Rain Oncology Inc, ReHeva Biosciences, Inc., Amgen, Novartis, RedCloud Bio, Parexel, Vitrac Therapeutics, LLC, Mythic Therapeutics, Instil Bio, Mirati Therapeutics Inc., Daiichi Sankyo, Inc., AstraZeneca, Precision Biologics, Inc, Promontory Therapeutics Inc., Palobiofarma SL, Regeneron Pharmaceuticals, Revolution Medicines, Inc., Cullinan Oncology, LLC, Iovance Biotherapeutics, Inc., Innate Pharma, among others.
Oncology Conference Coverage Services
DelveInsight’s Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Get in touch with us today to learn how we can provide AACR coverage exclusively for you at the AACR Meeting 2025
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Portfolio Management
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

